What is psoriasis?
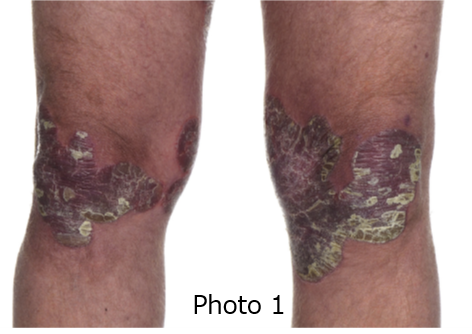
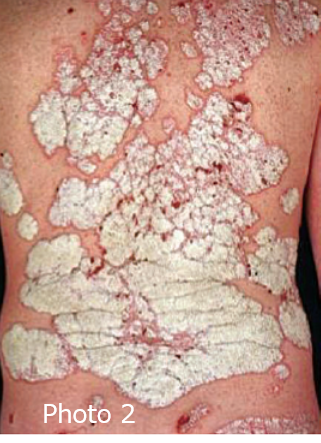
Psoriasis is a long-term (chronic) skin condition where one has elevated rough patches (plaques) on the skin. The color of the raised area may range from reddish (photo 1) to scaly silver (photo 2).
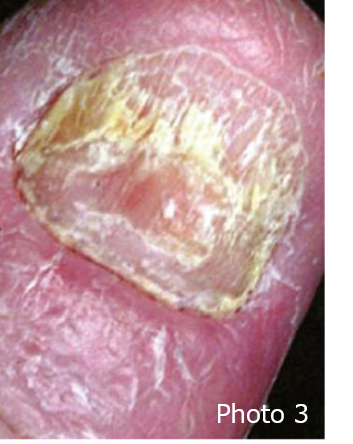
Psoriatic skin rash tends to develop on the body parts that are vulnerable to external physical stimuli such as the scalp, hairline, elbows and knees. The fingernails may develop deformity which is also a symptom of psoriasis (photo 3).
Psoriasis vulgaris, also known as ‘plaque psoriasis’ is the most common type of psoriasis where the aforementioned characteristic skin rash is the main symptom. According to the National Psoriasis Foundation and other sources, 2-3 % of total population worldwide suffer from psoriasis (roughly 1.5 % in African Americans, 3.6 % in Caucasians, and less than 1% in Asians). It tends to be more prevalent among male population than female and its onset is more common for people in their thirties to sixties.
Some patients with plaque psoriasis may develop joint symptoms like those seen in rheumatoid arthritis. This condition is known as ‘psoriatic arthritis.’
Psoriatic skin rash tends to develop on the body parts that are vulnerable to external physical stimuli such as the scalp, hairline, elbows and knees. The fingernails may develop deformity which is also a symptom of psoriasis (photo 3).
Psoriasis vulgaris, also known as ‘plaque psoriasis’ is the most common type of psoriasis where the aforementioned characteristic skin rash is the main symptom. According to the National Psoriasis Foundation and other sources, 2-3 % of total population worldwide suffer from psoriasis (roughly 1.5 % in African Americans, 3.6 % in Caucasians, and less than 1% in Asians). It tends to be more prevalent among male population than female and its onset is more common for people in their thirties to sixties.
Some patients with plaque psoriasis may develop joint symptoms like those seen in rheumatoid arthritis. This condition is known as ‘psoriatic arthritis.’
|
|
|
Photo 1: Medicine, Volume 41, Issue 6, 2013, pp. 334-340
Photo 2, 3: The Lancet, Volume 386, Issue 9997, 2015, pp. 983-994
Photo 2, 3: The Lancet, Volume 386, Issue 9997, 2015, pp. 983-994
What causes psoriasis?
The mechanism of the development of psoriasis is not completely understood. However, many recent studies have shed light to the concepts behind the disease and provided new findings to date.
The development of psoriasis is thought to be brought by environmental factors (such as smoking habits, stress, infections, obesity etc.) and genetic factors.
In general, skin cells (keratinocytes) start their ‘cell turnover’ at the bottom of the epidermis and make their way up to the surface of the skin, where the cells die and shed. This process takes about 28 days, or about a month.
In the psoriatic plaques, this ‘cell turnover’ becomes 10 times shorter than the healthy skin, taking only 3-5 days for keratinocytes to shed. This rapidity of ‘cell turnover’ forms a characteristic raised rash of psoriasis that peels in flakes (Figure 1)。
Interaction between skin cells (keratinocytes) and immune cells (especially ‘lymphocytes’ and ‘dendritic cells’) via substances called ‘cytokines’ has been shown to be a key to the development of psoriasis. Cytokines are a name of proteins secreted by these immune cells, and act as a communication tool to execute some action in the target immune cells. Among these cytokines, TNF-alpha, IL-17, and IL-23 are the key cytokines known to be actively involved in the development of plaque psoriasis.
The development of psoriasis is thought to be brought by environmental factors (such as smoking habits, stress, infections, obesity etc.) and genetic factors.
In general, skin cells (keratinocytes) start their ‘cell turnover’ at the bottom of the epidermis and make their way up to the surface of the skin, where the cells die and shed. This process takes about 28 days, or about a month.
In the psoriatic plaques, this ‘cell turnover’ becomes 10 times shorter than the healthy skin, taking only 3-5 days for keratinocytes to shed. This rapidity of ‘cell turnover’ forms a characteristic raised rash of psoriasis that peels in flakes (Figure 1)。
Interaction between skin cells (keratinocytes) and immune cells (especially ‘lymphocytes’ and ‘dendritic cells’) via substances called ‘cytokines’ has been shown to be a key to the development of psoriasis. Cytokines are a name of proteins secreted by these immune cells, and act as a communication tool to execute some action in the target immune cells. Among these cytokines, TNF-alpha, IL-17, and IL-23 are the key cytokines known to be actively involved in the development of plaque psoriasis.
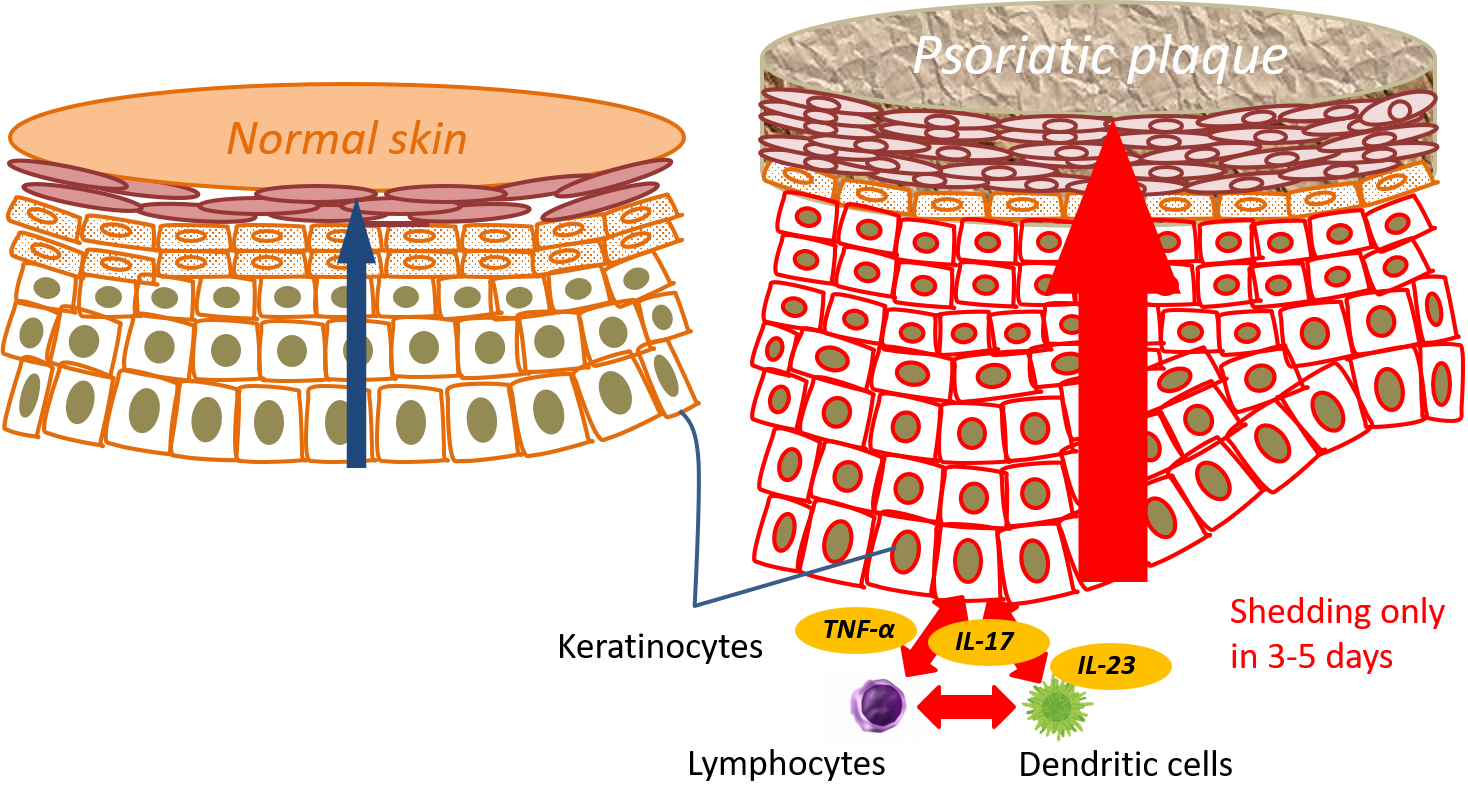
Figure 1. The image on the left shows a healthy skin where the cell turnover completes in about 28 days, whereas the one on the right shows psoriatic plaque where the cell turnover finishes in only 3-5 days.
The diagnosis of psoriasis and evaluation of its severity
A well-trained dermatologist can make a diagnosis of psoriasis merely by seeing the rashes if the lesion is typical. If the rashes are atypical, or leave the suspicion of other skin conditions, a skin biopsy under local anesthesia can be of help in making a correct diagnosis.
There are several ways to evaluate the severity of psoriasis:
①BSA (body surface area): a way to determine the severity by counting how many palms corresponds to the psoriasis rash area (one palm area is approximately 1 % of the body surface area) (Figure 2)
②PASI (Psoriasis Area and Severity Index): a method that uses a formula to calculate the degree of redness, elevation, roughness, and area of the rash (Figure 3).
More than 10% is regarded as severe in both measures.
There are several ways to evaluate the severity of psoriasis:
①BSA (body surface area): a way to determine the severity by counting how many palms corresponds to the psoriasis rash area (one palm area is approximately 1 % of the body surface area) (Figure 2)
②PASI (Psoriasis Area and Severity Index): a method that uses a formula to calculate the degree of redness, elevation, roughness, and area of the rash (Figure 3).
More than 10% is regarded as severe in both measures.
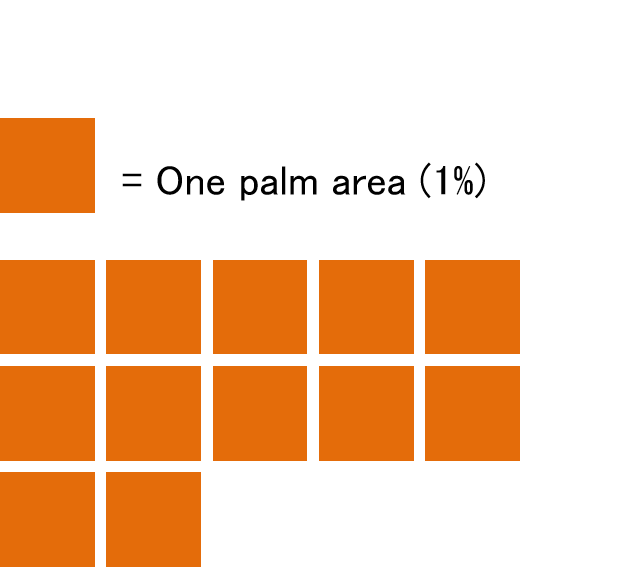
Figure 2. This is an image of BSA method. The BSA is 12 % if the rash area corresponds to 12 palms.

Figure 3. Image of the evaluation method using PASI. The rash's redness, elevation, degree of coarseness, and rash area are inclusively evaluated.
Psoriasis treatments
In psoriasis mainly showing skin eruptions (plaque psoriasis), treatment options are grossly divided into four categories:
①Topical therapy (treatment with ointment application)
②Phototherapy (treatment with therapeutic ultraviolet light)
③Oral medication (treatment with tablets or capsules)
④Injection therapy (treatment with injectable biological drugs)
Topical therapy is, in general, the most fundamental approach for plaque psoriasis. If it does not elicit a sufficient effect in controlling psoriatic plaques, phototherapy or oral medication can be a choice. Furthermore, if additional oral medication or phototherapy do not bring about expected improvement, injection therapy (biological drugs) can be a choice. (Note: phototherapy is NOT available at the clinic at this time)
We try to choose the best available treatment option considering the severity of the symptoms, efficacy of the ongoing treatment, and patients' life style.
①Topical therapy (treatment with ointment application)
②Phototherapy (treatment with therapeutic ultraviolet light)
③Oral medication (treatment with tablets or capsules)
④Injection therapy (treatment with injectable biological drugs)
Topical therapy is, in general, the most fundamental approach for plaque psoriasis. If it does not elicit a sufficient effect in controlling psoriatic plaques, phototherapy or oral medication can be a choice. Furthermore, if additional oral medication or phototherapy do not bring about expected improvement, injection therapy (biological drugs) can be a choice. (Note: phototherapy is NOT available at the clinic at this time)
We try to choose the best available treatment option considering the severity of the symptoms, efficacy of the ongoing treatment, and patients' life style.
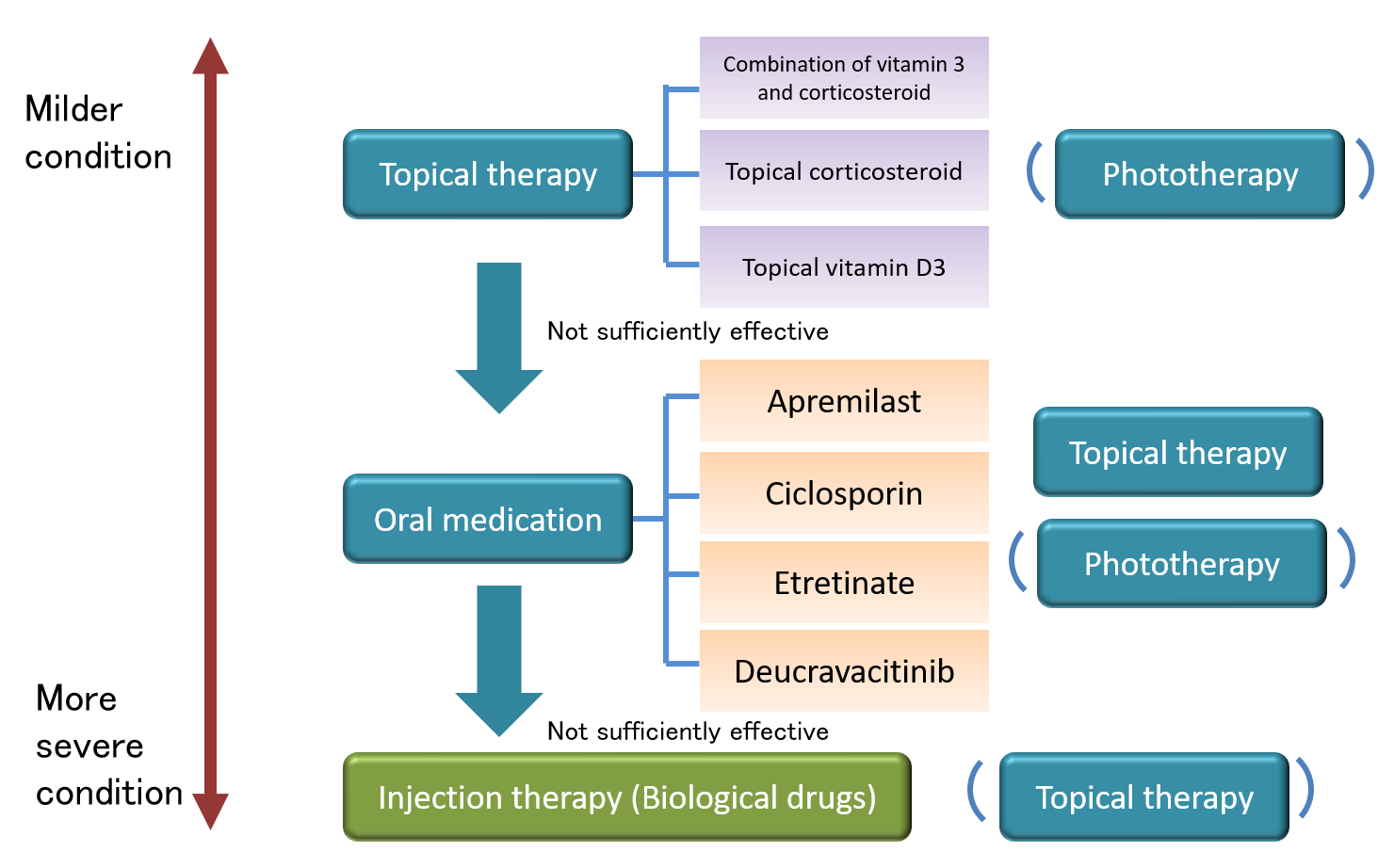
Figure 4. Overview of the Treatment Approaches for Plaque Psoriasis
Topical therapy (treatment with ointment application)
Topical therapy is the most fundamental approach for plaque psoriasis. The topical agent has two major components: topical corticosteroid, and active vitamin D3.
Topical corticosteroid acts with its potent anti-inflammatory effect. Active vitamin D3 subdues cell proliferation at the site of psoriatic plaques where they have increased cell turnover (Figure 1).
In recent years, topical combination medication of active vitamin D3 and corticosteroid such as Dovobet® or Marduox® is mainly used. And also, various forms of medication such as a shampoo, gel and foam in addition to ointment are now available and they broadened the choice of topical agents for the psoriatic lesion in different parts of the body based on the severity of the symptoms (for example, gels are applied onto the scalp while foams are applied onto the thick psoriatic plaque on the torso).
Topical corticosteroid acts with its potent anti-inflammatory effect. Active vitamin D3 subdues cell proliferation at the site of psoriatic plaques where they have increased cell turnover (Figure 1).
In recent years, topical combination medication of active vitamin D3 and corticosteroid such as Dovobet® or Marduox® is mainly used. And also, various forms of medication such as a shampoo, gel and foam in addition to ointment are now available and they broadened the choice of topical agents for the psoriatic lesion in different parts of the body based on the severity of the symptoms (for example, gels are applied onto the scalp while foams are applied onto the thick psoriatic plaque on the torso).
Oral medication (treatment with tablets or capsules)
Medications such as etretinate, ciclosporin, and apremilast can be used orally.
Tygason®(etretinate) takes effect by modulating abnormally potentiated keratinization of skin cells in psoriasis. Neoral® (ciclosporin), an immunosuppressant, subdues an overshoot of inflammation of psoriasis. Otezla® (apremilast), an immunomodulator, quieten the psoriatic lesion by interfering the interaction between skin cells and lymphocytes.
Sotyktu® (deucravacitinib) is a newly approved oral medication in September 2022. This medication blocks cytokines important in psoriatic plaque formation. For the introduction of Sotyktu® in the treatment of psoriasis, chest x-rays or chest CT scans, and blood tests including viral hepatitis infection check are required in advance.
Please be informed that, although we can offer the treatment with Sotyktu® at the clinic as an approved institute for the use of biological drugs in the treatment of psoriasis, chest x-rays or chest CT scans must be done at affiliated hospitals.
Tygason®(etretinate) takes effect by modulating abnormally potentiated keratinization of skin cells in psoriasis. Neoral® (ciclosporin), an immunosuppressant, subdues an overshoot of inflammation of psoriasis. Otezla® (apremilast), an immunomodulator, quieten the psoriatic lesion by interfering the interaction between skin cells and lymphocytes.
Sotyktu® (deucravacitinib) is a newly approved oral medication in September 2022. This medication blocks cytokines important in psoriatic plaque formation. For the introduction of Sotyktu® in the treatment of psoriasis, chest x-rays or chest CT scans, and blood tests including viral hepatitis infection check are required in advance.
Please be informed that, although we can offer the treatment with Sotyktu® at the clinic as an approved institute for the use of biological drugs in the treatment of psoriasis, chest x-rays or chest CT scans must be done at affiliated hospitals.
Injection therapy (treatment with injectable biological drugs)
Eleven injectable biological drugs (including one infusion drug) are now used in the treatment of psoriasis. Biological drugs, also known as biologics or biological agents are pharmaceutical drugs made from living sources or its products. Their use is considered if topical or oral conventional therapy were unable to elicit sufficient efficacy.
Interaction between skin cells (keratinocytes) and immune cells (especially ‘lymphocytes’ and ‘dendritic cells’) via substances called ‘cytokines’ has been shown to be very important in the development of psoriasis. Especially, TNF-alpha, IL-17, and IL-23 are the key cytokines known to be actively involved in the development of plaque psoriasis. Each biological drug is designed to block either one of these three cytokines and exert its effect.
Since the advent of biological drugs in 2010, the treatment of psoriasis has dramatically changed, and now we are in the era in which many patients with severe psoriasis can achieve almost plaque-free skin.
In Japan, biological drugs can be introduced at the institute that is approved by The Japanese Biological Association. Also, regular check-ups including blood tests, and imaging studies, and affiliation with a general hospital that is also approved for the use of biological agents, or with a hospital with board-certified respiratory physicians to deal with side-effects are regarded as necessary.
This clinic is an approved institute for biological drugs in the treatment of psoriasis, and we are committed to complying with these rules in our medical services.
In the introduction of, and continuous use of biological drugs, regular chest x-rays or chest CT scans, and blood tests including viral hepatitis infection check are required. Chest x-rays or chest CT scans must be done at the affiliated hospitals.
Treatment with biological drugs can be rather costly, and `High -Cost Medical Expense Benefit’ may apply to cover part of your medical expense if you have health insurance coverage in Japan and meet certain conditions.
We are committed to offering correct information regarding the medical subsidy system here in Japan, and practicing safe medical services.
Interaction between skin cells (keratinocytes) and immune cells (especially ‘lymphocytes’ and ‘dendritic cells’) via substances called ‘cytokines’ has been shown to be very important in the development of psoriasis. Especially, TNF-alpha, IL-17, and IL-23 are the key cytokines known to be actively involved in the development of plaque psoriasis. Each biological drug is designed to block either one of these three cytokines and exert its effect.
Since the advent of biological drugs in 2010, the treatment of psoriasis has dramatically changed, and now we are in the era in which many patients with severe psoriasis can achieve almost plaque-free skin.
In Japan, biological drugs can be introduced at the institute that is approved by The Japanese Biological Association. Also, regular check-ups including blood tests, and imaging studies, and affiliation with a general hospital that is also approved for the use of biological agents, or with a hospital with board-certified respiratory physicians to deal with side-effects are regarded as necessary.
This clinic is an approved institute for biological drugs in the treatment of psoriasis, and we are committed to complying with these rules in our medical services.
In the introduction of, and continuous use of biological drugs, regular chest x-rays or chest CT scans, and blood tests including viral hepatitis infection check are required. Chest x-rays or chest CT scans must be done at the affiliated hospitals.
Treatment with biological drugs can be rather costly, and `High -Cost Medical Expense Benefit’ may apply to cover part of your medical expense if you have health insurance coverage in Japan and meet certain conditions.
We are committed to offering correct information regarding the medical subsidy system here in Japan, and practicing safe medical services.
Table 1. A list of biological drugs used in the treatment of psoriasis
The following is a list of biological drugs used in the treatment if psoriasis as of 2013 in Japan. Except for Remicade, which is an infusion biologic drug, all are injectable medications. Target molecules for each biological drug is shown in red. Some drugs are self-injectable (the patient injects on his own), but others are not self-injectable and the healthcare provider will give an injection at the clinic.
|
BRAND NAME (Generic name)
|
Target molecules (more specific target molecules)
|
Injection interval*
|
Self-injection
|
|
TREMFYA (Guselkumab)
|
IL-23 (IL-23p19)
|
8 weeks
|
×
|
|
ILUMYA (Tildrakizumab)
|
IL-23 (IL-23p19)
|
12 weeks
|
×
|
|
SKYRIZI (Risankizumab)
|
IL23 (IL-23p19)
|
12 weeks
|
×
|
|
STELARA (Ustekinumab)
|
IL12/23 (IL-12/23p40)
|
12 weeks
|
×
|
|
COSENTYX (Secukinumab)
|
IL-17 (IL-17A)
|
4 weeks
|
〇
|
|
TALTS (Ixekizumab)
|
IL-17 (IL-17A)
|
2 to 4 weeks
|
〇
|
|
LUMICEF (Brodalumab)
|
IL-17 (IL-17RA)
|
2 weeks
|
〇
|
|
BIMZELX (Bimekizumab)
|
IL-17 (IL-17A/F)
|
4 to 8 weeks
|
×
|
|
HUMIRA (Adalimumab)
|
TNF-α
|
2 weeks
|
〇
|
|
CIMZIA (Certolizumab pegol)
|
TNF-α
|
2 to 4 weeks
|
〇
|
|
REMICADE (Infliximab)
|
TNF-α
|
8 weeks
|
× Infusion biologics
|
*In the initial treatment phase of biological drugs, many have what we call a ‘loading schedule’, where the injection is given at intervals shorter than those listed in the table, in order to increase the blood concentration of the drug. The injection interval listed in the table is the injection intervals after the ‘loading schedule’.
