About DUPIXENT® (Dupilumab)
Cytokines such as IL-4 and IL-13 are especially important in the development of eczema (atopic dermatitis). Secreted by helper T cells, these cytokines act on various cells resulting in reddish inflammatory skin seen in eczema.
An injectable medication for eczema, DUPIXENT® (dupilumab) is a monoclonal antibody medication (meaning that each antibody is identically structured at a molecular level) produced through biotechnology. DUPIXENT® prevents IL-4 and IL-13 from acting on their target cells (actually by blocking their receptors), and, as a result, subdues skin inflammation of eczema (Figure 1). Serial clinical trials have shown that the introduction of DUPIXENT® not only improves skin inflammation, and itchiness, it also contributes to more qualified sleep and better quality of life.
DUPIXENT® is also used in the treatment of asthma and part of sinusitis (inflammation of bony cavities in the face) since interfering IL-4 and IL-13 function in these conditions can also bring about therapeutic benefit.
An injectable medication for eczema, DUPIXENT® (dupilumab) is a monoclonal antibody medication (meaning that each antibody is identically structured at a molecular level) produced through biotechnology. DUPIXENT® prevents IL-4 and IL-13 from acting on their target cells (actually by blocking their receptors), and, as a result, subdues skin inflammation of eczema (Figure 1). Serial clinical trials have shown that the introduction of DUPIXENT® not only improves skin inflammation, and itchiness, it also contributes to more qualified sleep and better quality of life.
DUPIXENT® is also used in the treatment of asthma and part of sinusitis (inflammation of bony cavities in the face) since interfering IL-4 and IL-13 function in these conditions can also bring about therapeutic benefit.
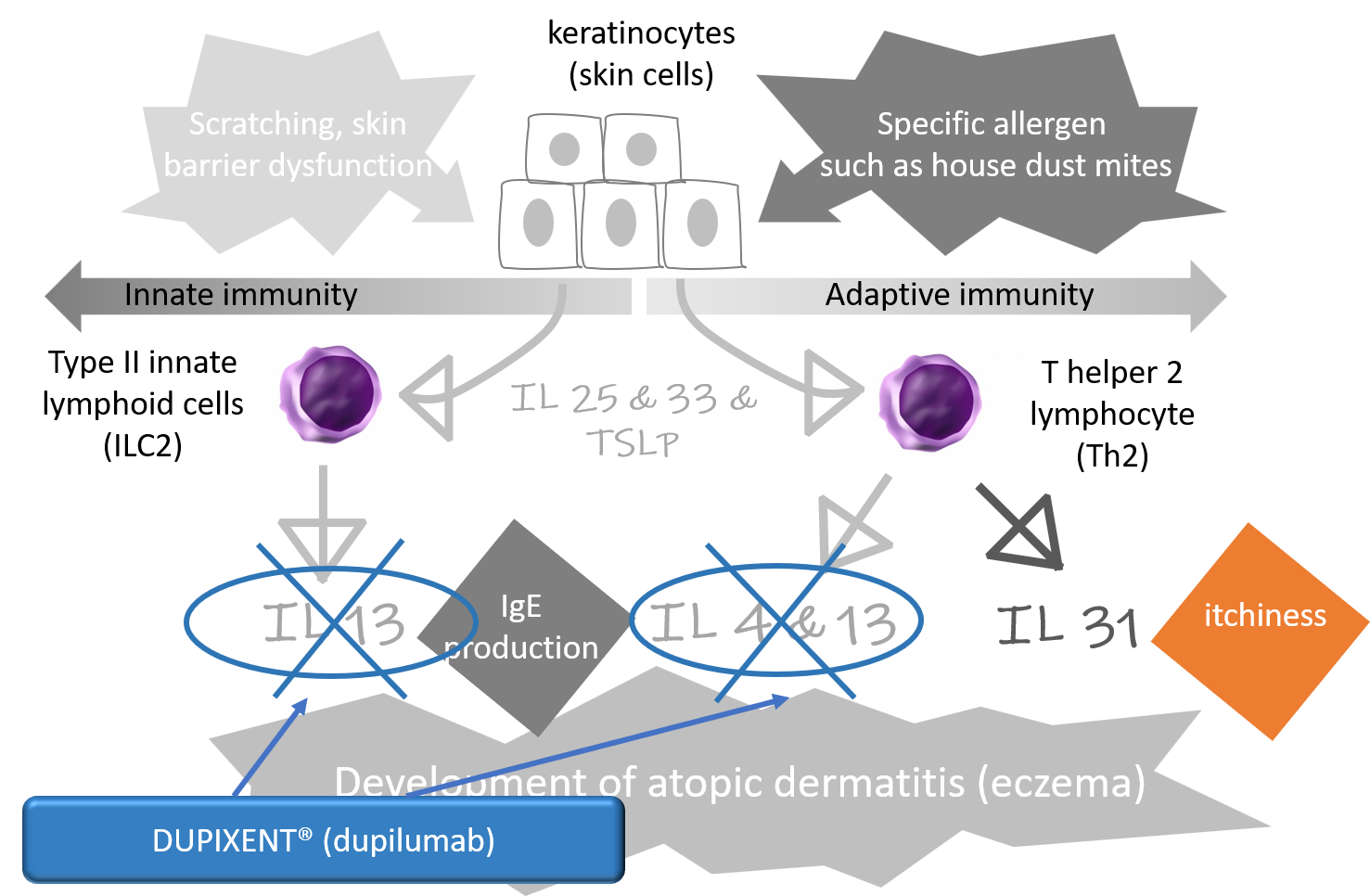
Figure 1. The site of action by DUPIXENT®
|
|
The left picture shows a single-dose prefilled pen which one presses against the injection site and the medication is automatically injected. The injection device of the left picture is a single-dose prefilled syringe with which one injects by pressing the plunger rod.
For whom treatment with DUPIXENT® is indicated?
In Japan, treatment with DUPIXENT is indicated for the patients aged 15 years and older with moderate-to-severe eczema (atopic dermatitis) whose condition is not adequately controlled despite appropriate topical therapies at least in the past 6 months.
Effectiveness of DUPIXENT® in the treatment of eczema
Effectiveness of DUPIXENT® (dupilumab) in the treatment of eczema was reported in some clinical trials. LIBERTY AD CHRONOS is one of the trials in which they evaluated the 16 weeks results of ① patients with topical corticosteroid application and dupilumab injection every other week vs. ②patients with topical corticosteroid application only (placebo) (Figure 1).
In this trial, an evaluation tool, EASI (Eczema Area and Severity Index) was used. EASI is one of the evaluation methods for eczema that scores the skin symptoms of eczema by scoring the rash area, redness, scratching, etc., and quantifies them in a composite manner. EASI-50, EASI-75, and EASI-90 respectively refer to the 50 %, 75 %, and 90% improvement of the EASI score from the baseline (that is, suppose your original skin manifestation makes 100, it would become 10 after EASI-90 achievement).
The result showed that the achievement rate of EASI-50, EASI-75, and EASI-90 at week 16 on dupilumab were 80.2 %, 68.9 %, and 39.6 % respectively as compared to those on placebo with the rates of 37.5 %, 23.2 %, 11.1 %, which proved the efficacy of dupilumab with statistically significant superiority.
In this trial, an evaluation tool, EASI (Eczema Area and Severity Index) was used. EASI is one of the evaluation methods for eczema that scores the skin symptoms of eczema by scoring the rash area, redness, scratching, etc., and quantifies them in a composite manner. EASI-50, EASI-75, and EASI-90 respectively refer to the 50 %, 75 %, and 90% improvement of the EASI score from the baseline (that is, suppose your original skin manifestation makes 100, it would become 10 after EASI-90 achievement).
The result showed that the achievement rate of EASI-50, EASI-75, and EASI-90 at week 16 on dupilumab were 80.2 %, 68.9 %, and 39.6 % respectively as compared to those on placebo with the rates of 37.5 %, 23.2 %, 11.1 %, which proved the efficacy of dupilumab with statistically significant superiority.
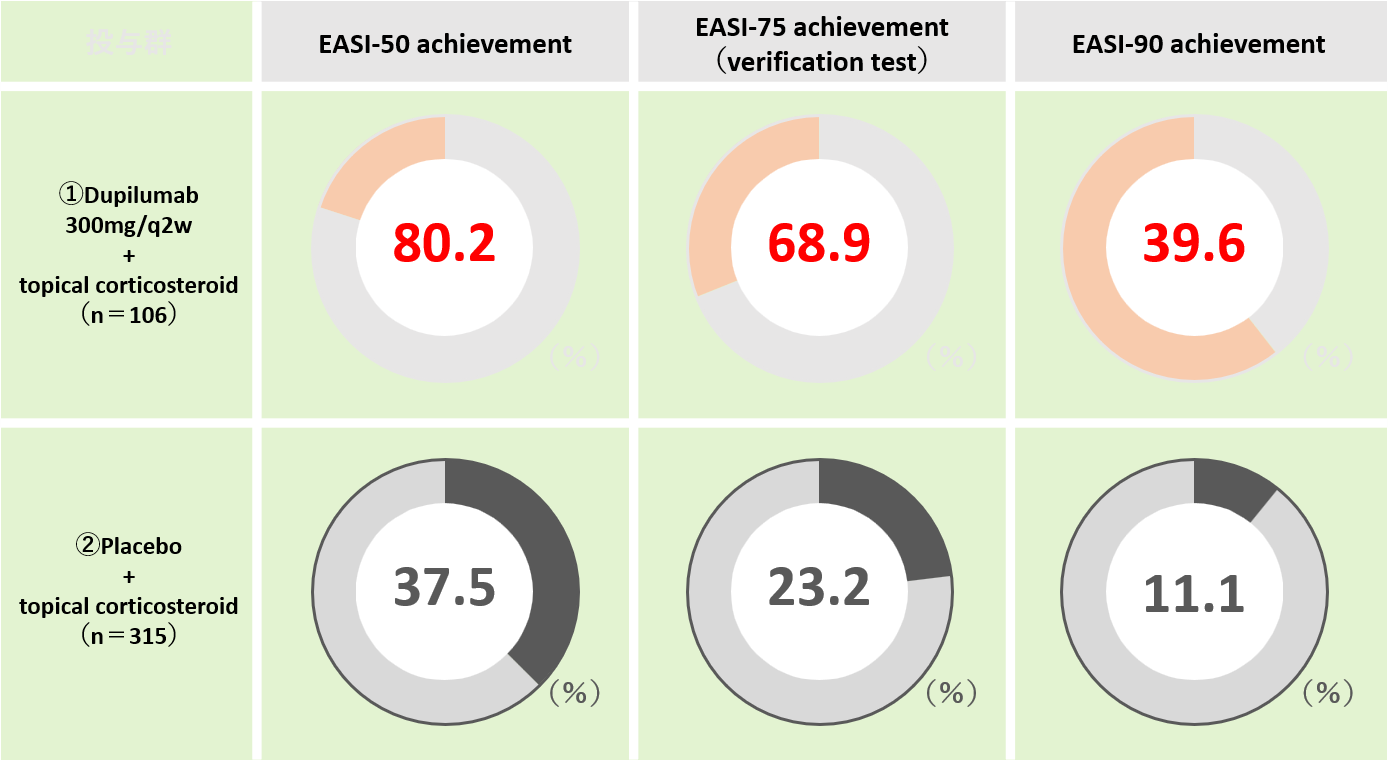
Blauvelt A et al. Lancet 2017;389:2287-2303
In addition to the skin evaluation using EASI by a dermatologist, we use self-evaluation tools for eczema, such as POEM (The Patient Oriented Eczema Measure) and ADCT (Atopic Dermatitis Control Tool) by a patient. Many patients show dramatic improvement in scores from the baseline at the clinic. For the detailed explanation of these scores, please refer to the section of ‘eczema’ in ‘our services’ on this website.
The flow of introduction of DUPIXENT® at the clinic
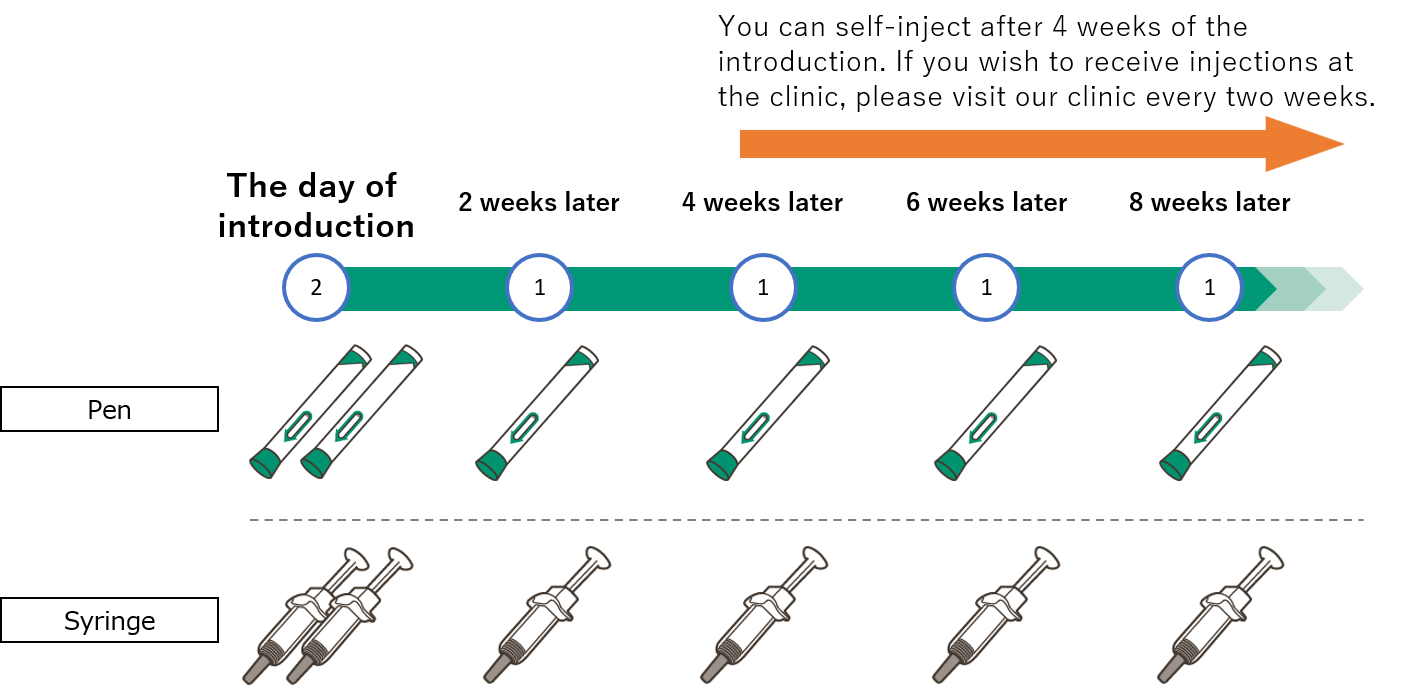
DUPIXENT® is an injection medication that is injected continuously once every two weeks. Self-injection at home is also possible after receiving instruction on self-injection. The following is the flow of introducing DUPIXENT® at our clinic.
Those who are receiving the treatment with DUPIXENT® for the first time
STEP 1. Consultation before introduction
Detailed interview especially on the history of treatment for eczema to date and assessment of severity of eczema will be conducted by a dermatologist. If the patient’s medical conditions overall indicate that he benefits from the treatment with DUPIXENT®, a discussion on the expected medical cost, options of the medical subsidy system available in Japan will be given as pieces of information. Then the date of introduction will be scheduled (DUPIXENT is not administered on the same day of the initial visit)
STEP 2. The day of introduction
Initial injections (two injections) of DUPIXENT® will be given at the clinic. If the patient wishes to self-inject, a healthcare provider at the clinic will give a first-time instruction on self-injection. If the patient does not wish to self-inject, injections will be given by a healthcare-provider at the clinic every two weeks thereafter.
STEP 3. Two weeks after the day of introduction
Second injection (one injection) will be given at the clinic. If the patient wishes to self-inject, a healthcare provider will give a second-time instruction of self-injection.
STEP4. Four weeks after the day of introduction
If the patient wishes to self-inject, DUPIXENT® will be prescribed (6 syringes or pens at one time at the maximum), and the next appointment will be scheduled (12 weeks later at the maximum).
Detailed interview especially on the history of treatment for eczema to date and assessment of severity of eczema will be conducted by a dermatologist. If the patient’s medical conditions overall indicate that he benefits from the treatment with DUPIXENT®, a discussion on the expected medical cost, options of the medical subsidy system available in Japan will be given as pieces of information. Then the date of introduction will be scheduled (DUPIXENT is not administered on the same day of the initial visit)
STEP 2. The day of introduction
Initial injections (two injections) of DUPIXENT® will be given at the clinic. If the patient wishes to self-inject, a healthcare provider at the clinic will give a first-time instruction on self-injection. If the patient does not wish to self-inject, injections will be given by a healthcare-provider at the clinic every two weeks thereafter.
STEP 3. Two weeks after the day of introduction
Second injection (one injection) will be given at the clinic. If the patient wishes to self-inject, a healthcare provider will give a second-time instruction of self-injection.
STEP4. Four weeks after the day of introduction
If the patient wishes to self-inject, DUPIXENT® will be prescribed (6 syringes or pens at one time at the maximum), and the next appointment will be scheduled (12 weeks later at the maximum).
For those who have already had DUPIXENT® introduced elsewhere
STEP 1. Initial consultation
Detailed interview especially on the history of treatment for eczema to date and assessment of severity of eczema will be conducted by a dermatologist. A discussion on the expected medical cost, options of the medical subsidy system available in Japan might be given depending on the situation. Then the date of prescription or injection will be scheduled (DUPIXENT is not administered on the same day of the initial visit).
STEP 2. Prescription or injection at the clinic
If the patient does self-inject, DUPIXENT® will be prescribed (6 syringes or pens at one time at the maximum). If the patient wishes to receive injections at the clinic, injections will be given every two weeks by a healthcare provider at the clinic.
Detailed interview especially on the history of treatment for eczema to date and assessment of severity of eczema will be conducted by a dermatologist. A discussion on the expected medical cost, options of the medical subsidy system available in Japan might be given depending on the situation. Then the date of prescription or injection will be scheduled (DUPIXENT is not administered on the same day of the initial visit).
STEP 2. Prescription or injection at the clinic
If the patient does self-inject, DUPIXENT® will be prescribed (6 syringes or pens at one time at the maximum). If the patient wishes to receive injections at the clinic, injections will be given every two weeks by a healthcare provider at the clinic.
Estimated expense of DUPIXENT®
|
First dose (2 injections)
|
Second dose (1 injection)
|
Self-injection (6 injections)
|
|
|
Cost of medication
|
117,550 yen
|
58,755 yen
|
352,530 yen
|
|
30% of fee (70% insurance coverage)
|
35,265 yen
|
11,755 yen
|
105,759 yen
|
|
20% of fee (80% insurance coverage)
|
23,510 yen
|
11,755 yen
|
70,506 yen
|
|
10% of fee (90% insurance coverage)
|
11,755 yen
|
5,878 yen
|
35,253 yen
|
The table is an estimate of the drug cost for pen-type Dupixent as of 2022. The amount to be paid at the reception for DUPIXENT® is shown in the table, and consultation and examination fees are charged separately. In addition, depending on your age, income and health insurance coverage, you may be eligible for ‘High-Cost Medical Expense Benefit’, ‘Additional Benefit System’, and other medical expense subsidy systems. For medical expense subsidy systems, please refer to ‘About medical expense subsidy systems’ on this website.
